Abstract
Despite the introduction of new therapies Systemic AL-amyloidosis (AL) remains incurable and the majority of patients will relapse. Autologous stem cell transplantation (ASCT) remains an important treatment option with improvement of organ function and survival in responding patients, however the toxicity of ASCT using conventional chemotherapy results in high morbidity and mortality limiting the application in this vulnerable patient group. We tested the use of a monoclonal anti-CD66 radiolabelled with yttrium-90, TLX66 (90Y-besilesomab) as the sole conditioning agent prior to ASCT in a phase I study in patients with AL. The study had full ethical and regulatory approvals (EudraCT 2015-002231-18; ISRCTN 13400668). The study consisted of three radiation activity levels, 30, 40 and 45 Mega Becquerels (MBq) per kg body weight with 3 patients treated at each activity level. Patients entered the study after fulfilling entry requirements and with informed consent. Prior to receiving the 90Y-besilesomab organ dosimetry and biodistribution were determined using the same anti-CD66 radiolabelled with indium-111 (111In-anti-CD66) with sequential gamma camera imaging. Estimated absorbed radiation to critical organs was determined from an established dosimetry model, organ radiation dose expressed in Gray (Gy). Radiation dose limits of 45Gy for the bone marrow and 15Gy for the liver were used as safety measures.
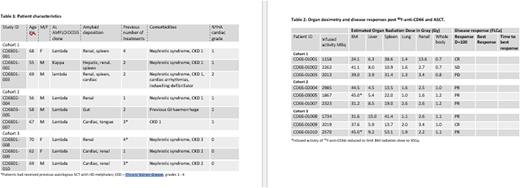
Table 1 summarises patient details. The median previous lines of treatment were 2 (range 1-5) including three who had previous ASCT using high dose melphalan. The primary end-point was treatment related toxicity as determined using CTCAE v4.0. Haematological toxicities (cytopenia) were excluded as adverse events (AEs) unless considered life-threatening. Secondary end-points: Disease response determined by changes in clonal free light chain assay (FLCa), malignant plasma cell percentage in BM as determined by multi-colour flow cytometry; cardiac toxicity from NT-proBNP assay; overall survival and time to disease progression; biodistribution and dosimetry SPECT-CT imaging; engraftment using the European Blood and Marrow Transplant (EBMT) criteria for neutrophil and platelet engraftment.
Results: Ten patients underwent dosimetry with 111In-anti-CD66, 9 received the 90Y-besilesomabwith infused activity determined by study cohort and patient body weight; one patient failed dosimetry with excessive hepatic uptake, two received reduced activity to keep the marrow dose within the 45Gy limit. There were no serious adverse events (SAE's) in the trial. There were 3 grade 4 AE's (mainly cytopenias during transplantation) and no SUSAR's were reported. A total of 41 grade 1-4 AE's were recorded. There were no transplant related deaths. All patients became profoundly cytopenic consistent with bone marrow suppression due to the targeted radiation. All patients engrafted with median neutrophils recovery by D+13 and platelets by D+11. Importantly, no patients experienced any mucositis or therapy related diarrhoea. All maintained oral nutrition. Only two patients experienced a fever post-transplant, neither were life-threatening. Table 2 summarises the estimated absorbed radiation dose to critical organs and disease responses. Of the 9 patients treated with 90Y-besilesomaband ASCT 7 had haematologic responses with 2 complete responses (CR), 5 partial responses (PR), 1 stable disease (SD) and 1 progressive disease (PD) as determined. Six of 8 evaluable patients showed a reduction in percentage of malignant plasma cells in the BM. The study collected data up to D+100 post-transplant but 2 patients showed continuing slow reduction in clonal FLCa beyond D+100 with 1 achieving a CR 5 months post-transplant. No radiation dose limiting toxicity was seen. No consistent changes were seen in NT-proBNP. Importantly no cardiac AEs were recorded. All patients are alive. The long term follow up data will be presented.
Acknowledgement: The native anti CD66 MAb (Besilesomab) was provided by Telix Pharmaceuticals. TLX66 (90Y-besilesomab) was acquired by Telix Pharmaceuticals in 2021.
Disclosures
Jessel:Telix Pharmaceuticals: Current Employment. Wechalekar:Alexion: Honoraria, Other: Travel Support; Attralus: Honoraria; Janssen: Honoraria, Other: Travel Support; Takeda: Other: Travel Support; GSK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal